Fibrinogen Early In Severe Trauma studY II (FEISTY II) is a phase III randomised clinical trial with the primary aim to determine whether earlier replacement of fibrinogen – a key clotting factor – using FC improves outcomes for patients with major haemorrhage following trauma, compared to Cryo.
Information for Investigators
FEISTY II – Investigating Fibrinogen Replacement in Traumatic Haemorrhage

FEISTY II will be coordinated by the Australian and New Zealand Intensive Care Research Centre (ANZIC-RC), is endorsed by the Blood Synergy Investigators, the Australian and New Zealand Intensive Care Society Clinical Trials Group (ANZICS-CTG), the Australian College of Emergency Medicine Clinical Trials Network (ACEM-CTN), the Australian and New Zealand Trauma Society (ANZTS) and Australian and New Zealand Association for the Surgery of Trauma (ANZAST) and will address a key evidence gap in the management of severely injured trauma patients with major haemorrhage.
Fibrinogen replacement for haemorrhage following trauma is a National Blood Authority identified key evidence gap. The trial compares the two available blood products commonly used for fibrinogen replacement worldwide during major haemorrhage, with the aim to inform clinicians and policy makers about how best to deliver blood product support to improve outcomes for individual patients following major trauma, and how best to manage our national blood supply
Specifics of the trial
Trial Aims
Fibrinogen replacement for haemorrhage following trauma is a National Blood Authority identified key evidence gap. The trial compares the two available blood products commonly used for fibrinogen replacement worldwide during major haemorrhage, with the aim to inform clinicians and policy makers about how best to deliver blood product support to improve outcomes for individual patients following major trauma, and how best to manage our national blood supply
Trial Design
A prospective phase III, multi-centre, randomised, controlled, two arm parallel, open-label trial evaluating the effect of FC compared to Cryo in severely injured bleeding adult trauma patients with major haemorrhage and hypofibrinogenemia.
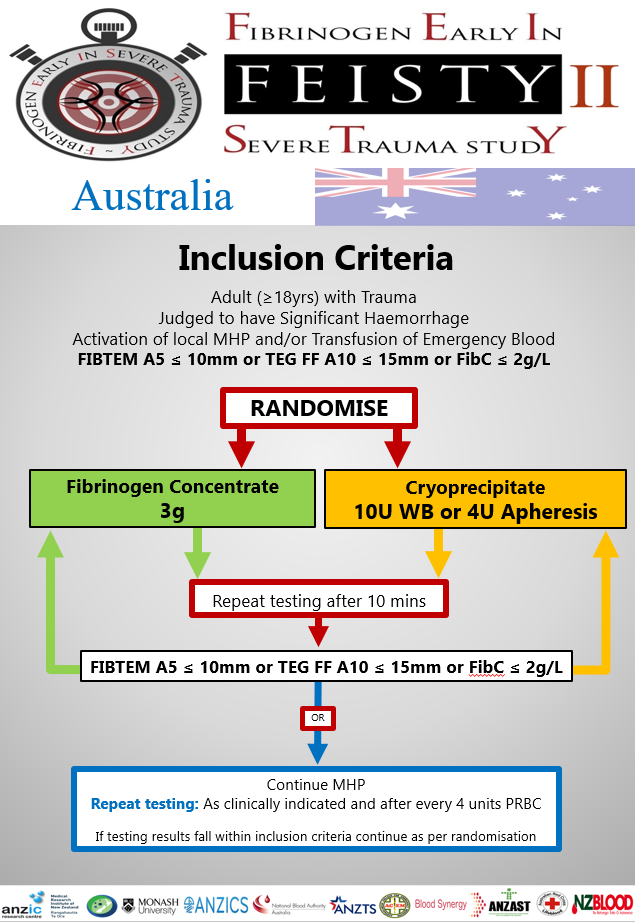
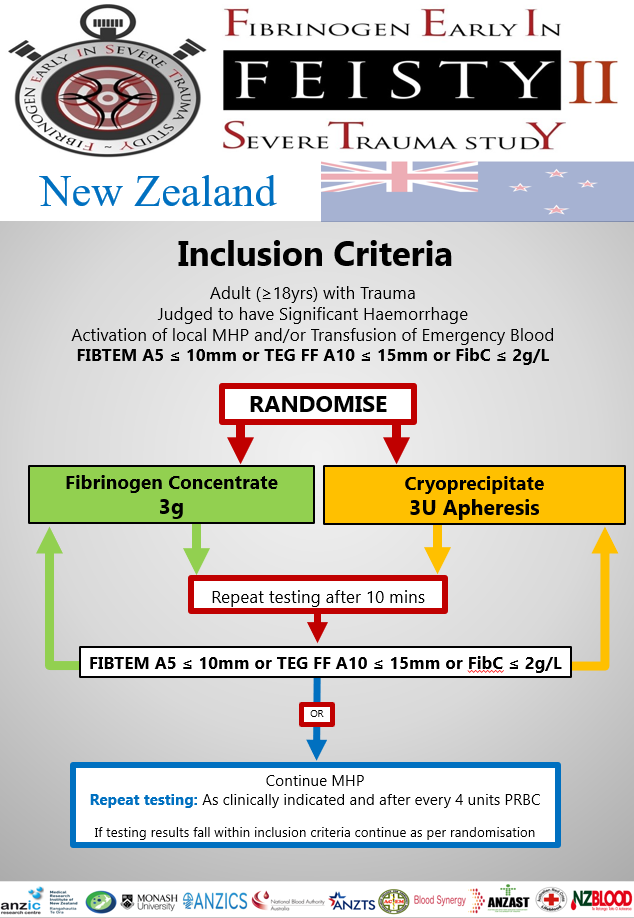
Inclusion Criteria
– Adult patient affected by trauma (age ≥ 18 years) AND
– Judged to have active haemorrhage by treating clinician AND
– Activation of local MHP and/or Transfusion of Emergency Blood Products AND
– FITBEM A5 ≤ 10mm or TEG FF A10 ≤ 15mm or Clauss Fib 2g/l
Exclusion Criteria
– Injury judged incompatible with survival
– Randomisation unable to occur within 6 hours of presentation to hospital
– Known pregnancy
– Known genetic or drug induced coagulation disorder
– Known objection to blood products
– Dedicated prior fibrinogen replacement (this presentation)
– Severe pre-existing physical or mental disability or severe co-morbidity that may interfere with the assessment of outcome
– Participation in a competing study
Outcome Measures
Primary Outcome
Number of days alive out of hospital (at home) at 90 days (DAOH90)
Secondary Outcomes
- Number of RBC units at 24 hours after randomisation
- Fibrinogen levels post-intervention
- All-cause mortality at 6hrs, 24hrs, 28 and 90 days from randomisation
- Days out of hospital in survivors at 90 days
- Death from haemorrhage at 6hrs and 24hrs from randomisation
- ICU and hospital (acute) free days
- Ventilation-free days up to day 28 from randomisation
- Denver MOF Score up to day 28 from randomisation
- Fibrinogen levels over time (if part of standard care)
- Symptomatic thromboembolic (arterial and venous) complications
- EQ5D-5L at D90, 6 and 12 months from randomisation
- WHODAS-2.0 at D90, 6 and 12 months from randomisation
- GOSE in patients with moderate to severe TBI at 12 months from randomisation
Collaborators and Sponsors
Participating Sites











We are fortunate to be supported by funding grants from
Our collaborators include








Research Support


Industry support for the FEISTY study is limited to materials only with no input to the design and conduct of the study.
Copyright © 2016 FEISTY